Liquid crystals are an industrially important class of compounds as they are used in a variety of displays for watches, calculators, and lap-top computers. The utility of liquid crystalline molecules arises because they can be oriented using an electric or magnetic field, and this reorientation drastically changes the way that light interacts with the molecules. The ability to be oriented is due to the fact that these compounds possess intermolecular interactions that are weaker than those found in solids and stronger than those found in liquids.
Unlike typical substances, which melt directly from a solid to a liquid, thermotropic liquid crystals undergo a phase transition from a solid to an intermediate phase (mesophase) before melting to an isotropic liquid upon further heating. In fact, many liquid crystals exhibit multiple mesophases. Liquid crystalline behavior is often observed for rod shaped molecules that possess a rigid core (typically consisting of aromatic rings), which provides order, and a flexible tail (such as an alkyl group), which provides disorder.
My research focuses on the synthesis and analysis of new organic liquid crystals. In general, when examining a particular class of molecules for liquid crystalline behavior, a homologous series is prepared by systematically varying the length of the alkyl group. This is done because small changes in structure often lead to changes in liquid crystalline behavior. My students and I use standard organic and organometallic synthetic techniques to prepare our compounds, and NMR and IR spectroscopy to structurally characterize them. To study the liquid crystalline behavior, we use a polarizing microscope equipped with a heating stage and differential scanning calorimetry (DSC).
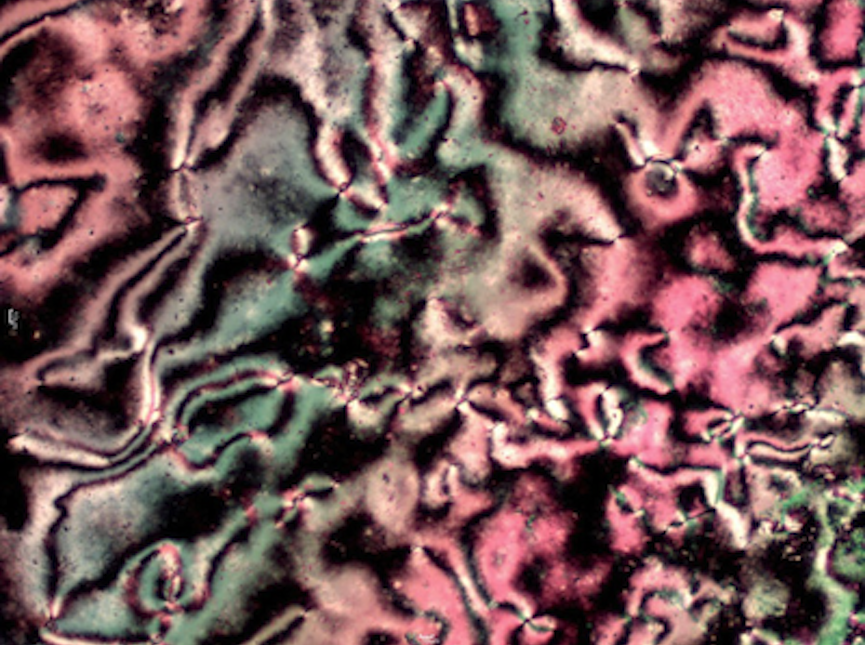
Nematic phase of an oxadiazole based liquid crystal as viewed by polarizing microscopy