Joe Ewers ’21 looks to cow eyes to answer questions about the causes of neurological diseases
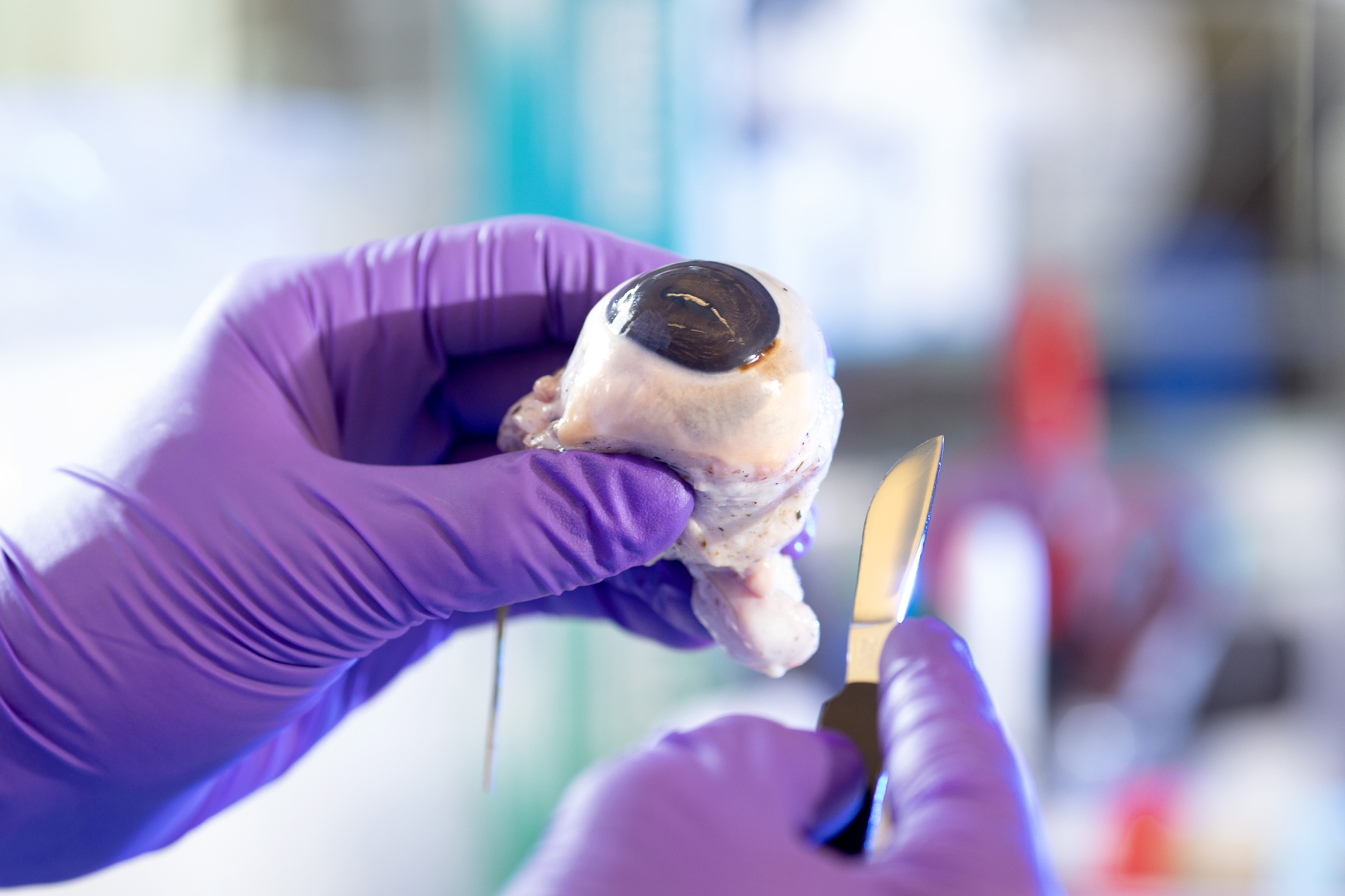
With a pair of surgical scissors in one hand and a cow eyeball in the other, Joe Ewers ’21 carefully cuts into the organ and removes the lens.
The clear lump doesn’t look like much. It’s about the size and shape of a flattened marble, but within its structure is a protein that could hold answers as to what causes degenerative neurological conditions such as Parkinson’s disease.
Joe is working on his summer research project—one of more than 80 being completed by Puget Sound students in the sciences and humanities through summer research grants after a rigorous and comprehensive application process. Under the guidance of Assistant Professor of Chemistry Megan Gessel, who has been studying the link between the protein Aquaporin-0, oxidation, and neurological disorders for years, Joe will log more than two months in the labs of Thompson Hall this summer. His work aims to purify and collect Aquaporin-0, which will then be inserted into an oxidized membrane created by fellow biochemistry student and summer researcher Walker Shibley-Styer ’20. “We want to see how the Aquaporin oxidizes,” Joe says.
In recent years, researchers have found that the breakdown of lipids—molecules found in the membranes of living cells—plays a huge role in the development of several diseases, including degenerative neurological diseases. By studying how proteins are affected by this oxidation and subsequent breakdown, Joe and Walker are hoping to contribute to the research that’s helping scientists find ways to slow down or counteract this oxidation and breakdown, which could ultimately lead to cures for debilitating diseases like Parkinson's.
“It's exciting for me, because I really enjoy seeing where reactions and concepts I learn in lecture have real-life applications,” Joe says. “It’s a great opportunity to be able to do research in this field because it is showing me a lot of what I could do in the future.”
Scroll through the photos below to see exactly what Joe's research process entails.
First, Joe uses a scalpel and surgical scissors to cut the eye and remove the lens.
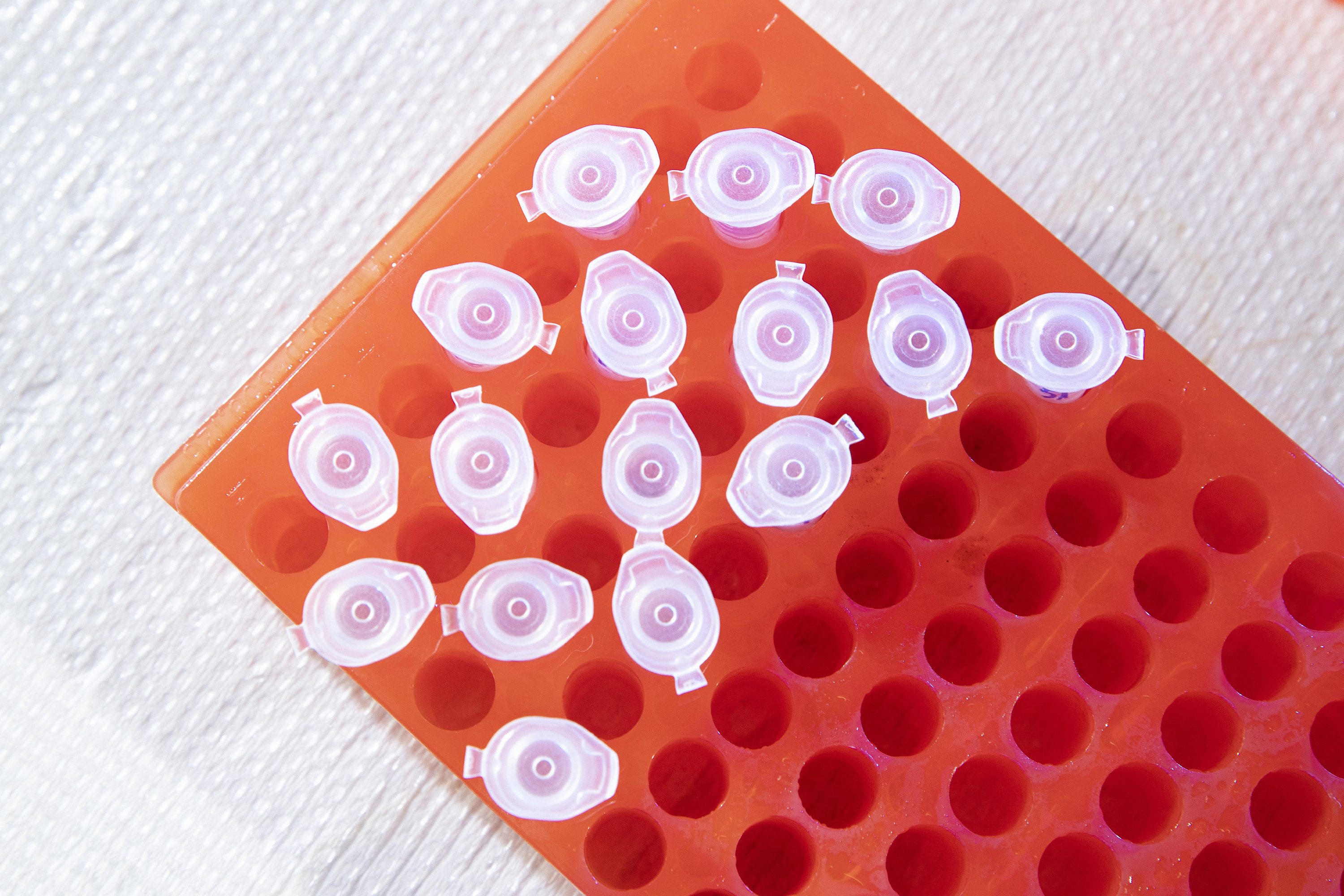
Then he cuts the lens into four pieces and places each segment in an Eppendorf tube.
He adds two chemicals to the tube: one to dissolve the Aquaporin-0 protein in the lens (Dodecylmaltocide) and one to help the solution mix together uniformly.
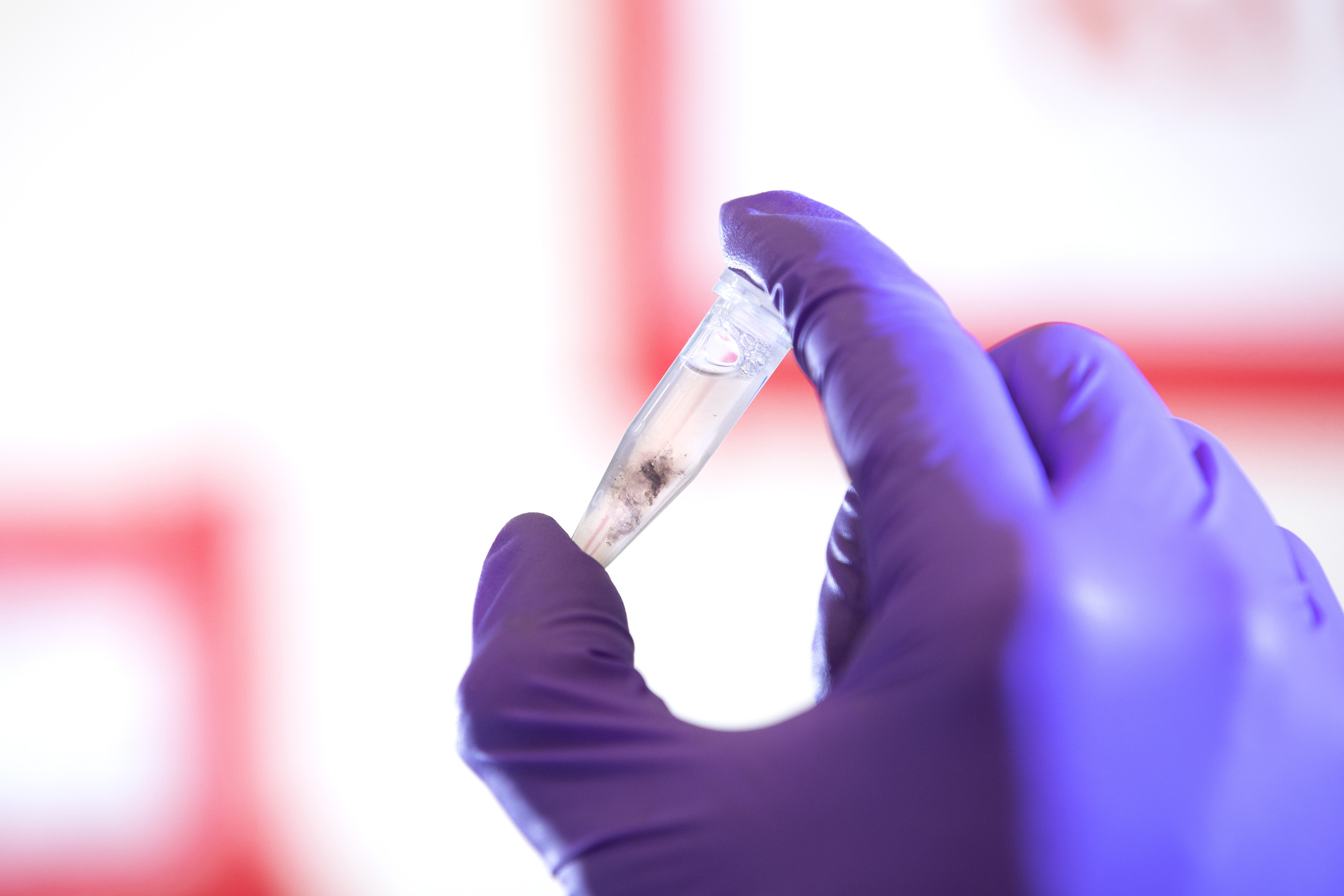
The lens-and-chemical solution is placed into a machine to be heated and mixed for 30 minutes to allow the chemicals to break down the lens.
After 30 minutes, the solution is placed in a centrifuge to separate the protein from any larger particles that may have made their way into the solution from the eye.
The clear liquid containing the dissolved Aquaporin-0, which now sits at the top of the tube, is collected and transferred to another tube. That mixture is taken to a machine called an FPLC—fast protein liquid chromatography—that will purify the solution and collect the purest Aquaporin-0 possible.
Joe will see readouts on the computer screen that tell him whether the sample is pure enough to move forward, or if he needs to start over. If the sample is at least 90% pure, it will be inserted into the membrane made by Walker and further studied.